25 years of groundbreaking research — an annotated history
PREFACE: PLANT TEACHER
Ancient peoples attuned to ecological subtleties referred to certain consciousness-altering plants and fungi as “teachers.” What has cannabis taught humankind?
Long before the written word, cannabis figured prominently in the shamanistic traditions of many cultures, which found uses for virtually every part of the plant. The stalk provided fiber for cordage and cloth; the seeds, a rich source of protein and essential fatty acids, were eaten as food; and the roots and resinous flower tops were used in medicinal and ritual preparations.
What accounts for the herb’s broad and enduring appeal? Scientific efforts to pinpoint the psychoactive ingredients that cause the mild euphoria beloved by cannabis enthusiasts began in the 19th century. But investigators were stymied by the complex, lipophilic (oily) nature of the plant, which required sophisticated technology to probe and parse.
A key turning point for modern cannabis research came in 1964, when Israeli scientists Raphael Mechoulam and Yechiel Gaoni isolated and identified tetrahyrdocannabinol (THC) as the high causer. Mechoulam also elucidated the chemical structure of several other cannabis components, including cannabidiol (CBD), an intriguing, non-intoxicating molecule. He called these unique botanical compounds “cannabinoids” and likened the plant to “a pharmacological treasure trove.”
The buzz about THC, the queen bee of cannabinoid pharmacology, was the main impetus for scientists who sought to understand how marijuana conferred its psychoactive effects. What happens in the brain that makes people feel high? Or hungry? Or calm? Or slightly less encumbered by life’s difficulties? Animal studies focusing on THC provided a foundation for investigating its mechanism of action on a molecular level. Another quarter of a century would pass before cannabis, the plant teacher, led researchers to one of the great scientific discoveries of all time – actually, a series of discoveries – that revealed the existence and inner workings of a protective, body-wide regulatory system activated by cannabinoid compounds.
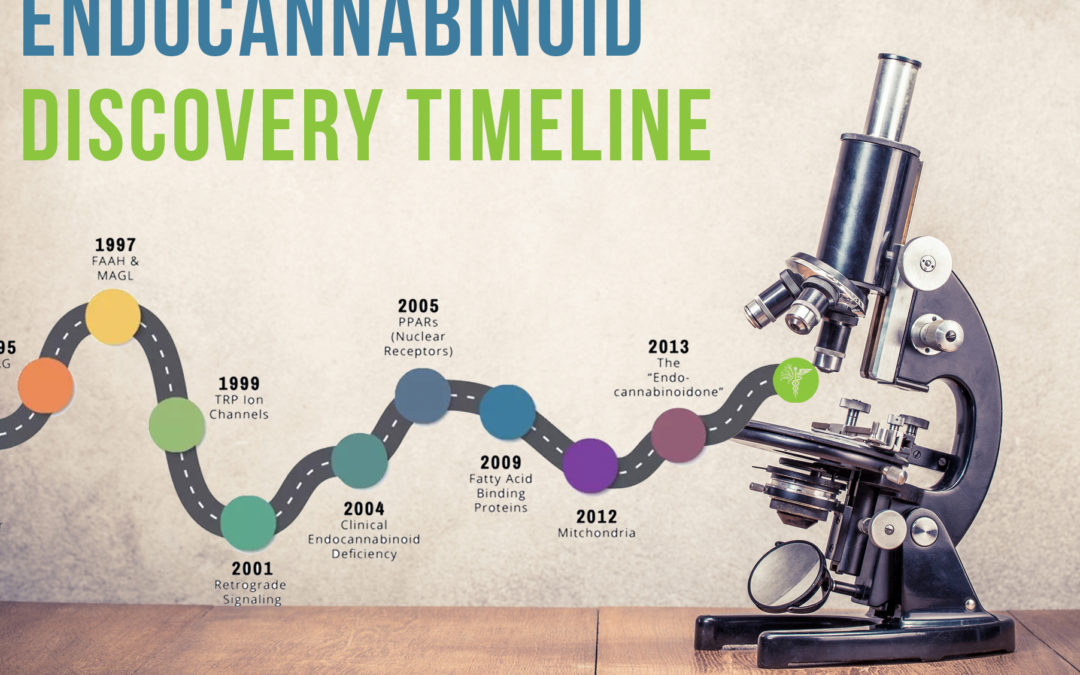
ENDOCANNABINOID DISCOVERY TIMELINE

PART 1: THE CANONICAL ENDOCANNABINOID SYSTEM
1988: CB1 RECEPTOR
The big breakthrough came in 1988, when scientists at the St. Louis University Medical School determined that a rat’s brain has receptor sites – specialized protein molecules embedded in cell membranes – that are activated by THC. Initially identified by Professor Allyn Howlett and her graduate student William Devane, and cloned two years later, this cannabinoid receptor, dubbed “CB1,” turned out to be far more abundant in the mammalian brain than any other G-protein-coupled receptor (GPCR).
Nearly half of all U.S.- approved pharmaceuticals target GPCRs, which comprise a super-family of over 800 different human receptors that share the same basic protein structure – hundreds of amino acids strung together in a crumpled chain, crisscrossing the cell membrane seven times. CB1 receptors are concentrated in the mammalian brain and central nervous system. Subsequent research showed that CB1 receptors are also present to a lesser extent in the gut, skin, and various internal organs. All animals with a spinal cord (and going back even earlier to the ancient sea squirt) have CB1 receptors. CB1 signaling would prove to be critical for regulating numerous physiological processes, including the body’s stress response and how we experience pain.
The discovery of the CB1 receptor would have huge implications for nearly every area of medical science. It opened the floodgates of research into our innate cannabinoid biology. Why do we have receptors that are capable of responding to plant cannabinoids such as THC? Scientists realized there had to be an endogenous, THC-like compound, our inner cannabis, so to speak, that signaled through these receptors. The search was on for CB1’s internal trigger.
1992: ANANDAMIDE
Enter N-arachidonoylethanolamine, the first endogenous cannabinoid neurotransmitter identified by scientists. (A neurotransmitter is a chemical that nerve cells use to send signals to other neurons.) In 1992, a trio of researchers at Hebrew University in Jerusalem – Raphael Mechoulam, William Devane, and Lumir Hanus – isolated a novel lipid neurotransmitter that binds with the CB1 receptor in pig brain tissue. They called it “anandamide,” Sanskrit for bliss, a word suggestive of its mood-altering effects.
Although anandamide and THC don’t share a similar molecular structure, they behave in a similar way when they bind to the CB1 receptor, somewhat like a key fitting into a lock. Anandamide, the endocannabinoid, and THC, the phytocannabinoid, are both signaling molecules (ligands) that turn on CB1, initiating a cascade of changes within cells that regulate an astonishing range of physiological functions, including appetite, mood swings, glucose metabolism, pain perception, even fertility. High levels of anandamide are crucial for ovulation, and fluctuations of anandamide levels during the gestational cycle can affect fetal development.
Cells create anandamide “on-demand,” whenever our bodies need to stay even keel during stressful interludes. Subsequent studies would show that physical exercise boosts anandamide levels, resulting in the “runner’s high.” By binding to CB1, anandamide protects neurons and facilitates neurogenesis, the creation of new brain cells in adult mammals. Every animal with a nervous system produces anandamide.
1993: CB-2 RECEPTOR
Scientists identified a second type of cannabinoid receptor – “CB2” – which is present throughout the immune system, the peripheral nervous system, metabolic tissue, and in many internal organs. Initially reported in Nature in 1993, this discovery shed new light on how cannabinoid signaling regulates inflammation and why cannabinoid therapy could be a helpful treatment for a raft of autoimmune diseases. Aberrant CB2 receptor signaling is implicated in metabolic syndrome, peripheral neuropathy, insulin resistance, liver disease, and other inflammatory conditions.
CB2 receptors are found in all immune cells, including microglia and astrocytes, which modulate immune function in the brain. For the most part, however, CB2 receptors are expressed much less than CB1 in the central nervous system. But CB2 is significantly upregulated (kicks into high gear) in response to a brain injury or a neurodegenerative condition, such as Alzheimer’s or multiple sclerosis.
THC stimulates both types of cannabinoid receptors. However, when THC binds to CB2, it does not trigger the psychoactive high that cannabis is known for because CB2 receptors are not concentrated in the brain. THC binding to CB1, the abundant central nervous system receptor, causes the high. Consequently, researchers set their sights on healing without the high by developing drugs that stimulate the CB2 receptor, while bypassing CB1. But anandamide, the endocannabinoid that binds to CB1, actually has very little binding affinity for CB2 – which means there must be another natural compound, an endogenous ligand, produced by the body that activates CB2 receptors.
1995: 2-AG
Found in canine gut tissue, 2-Arachidonoylglycerol – or 2-AG for short – was identified as an endocannabinoid by Dr. Mechoulam and his team, and also by Japanese scientists, in 1995. Compared to anandamide, 2-AG proved to be more potent, more prevalent, and more broadly expressed throughout the body. 2-AG levels in the human brain are approximately 170 times higher than those of anandamide, and 2-AG binds efficiently to both cannabinoid receptors, CB1 and CB2.
Anandamide and 2-AG are both lipid neurotransmitters that signal all over the brain and body to help maintain internal homeostasis amidst a barrage of ever-changing environmental inputs. As the principal endogenous ligand for both CB1 and CB2, 2-AG plays a major role in regulating immune function. It reduces the expression of pro-inflammatory cytokines and reins in overactive immune cells. 2-AG levels in the brain surge after a head injury or a stroke.
Like anandamide, 2-AG is involved in modulating a wide range of mental and physiological processes. While they are similar and complementary in many respects, there are specific functional differences between the two endocannabinoids. Anandamide and 2-AG both protect cells against oxidative damage, and both compounds are adaptive in response to stress – but in distinct ways. And they are created and deactivated by different metabolic enzymes.
1997: METABOLIC ENZYMES – FAAH AND MAGL
Endocannabinoids are born and broken down by various biosynthetic and catabolic enzymes. Thanks to these metabolic enzymes, endocannabinoids are made when needed and then degraded after serving their purpose. Anandamide is broken down by FAAH [fatty acid amide hydrolase], while 2-AG is deactivated primarily by MAGL [monoacylglycerol lipase]. The molecular structure of FAAH was characterized by Ben Cravatt at the Scripps Research Institute in 1996, and the following year Italian scientists identified MAGL as a key degradative enzyme for 2-AG.
Metabolic enzymes regulate endocannabinoid activity by controlling anandamide and 2-AG levels. Because anandamide & 2-AG degrade rather quickly, blocking their enzymatic metabolism – by inhibiting FAAH or MAGL – can elevate endocannabinoid levels and extend cannabinoid receptor signaling, with consequent neuroprotective benefits. Variations in the genes that code for FAAH and MAGL are associated with divergent health outcomes; too much of either enzyme can deplete endocannabinoid tone, resulting in what some would call a “weak constitution.”
The cloning of FAAH and MAGL marked a decade since the momentous discovery of the CB1 receptor, which really got the ball rolling in terms of cannabinoid science. The two cannabinoid receptor subtypes along with anandamide, 2-AG, and their biosynthetic and degradative enzymes, comprised the basic components of the canonical or “classical” endocannabinoid system, which modulates most biological functions. The endocannabinoid system plays a pivotal role in maintaining a healthy, stable environment inside the body, despite fluctuating external inputs and stressors. In the years ahead, new research would deepen our understanding of this ubiquitous lipid signaling ensemble.
PART 2: FORAGING THE NEURONAL FOREST
1998: ENTOURAGE EFFECT
The phrase “entourage effect” first appeared in a July 1998 science paper by S. Ben-Shabat and several colleagues. Published in the European Journal of Pharmacology, the article focused on 2-AG and “a novel route for molecular regulation of endogenous cannabinoid activity.” The authors reported that 2-AG’s binding affinity for CB1 and CB2 was enhanced by the presence of other endogenous lipid compounds that were not, strictly speaking, part of the canonical cannabinoid framework. A subsequent paper that year in the same journal discussed “the effects of ‘entourage’ compounds on the activities of anandamide and 2-arachidonoyl glycerol.”
A scientific phrase intended as a reference to the holistic, interactive underpinnings of the endocannabinoid system was subsequently applied to the complex chemical make-up of herbal cannabis. Just as endocannabinoids don’t act in isolation, neither do plant cannabinoids. The effects of THC and CBD are influenced by dozens of aromatic terpenes, flavonoids, and minor cannabinoids that may be present in a given cultivar. Each of these compounds has specific medicinal attributes, but when combined they create an entourage or ensemble effect so that the therapeutic impact of the whole plant (flower or essential oil) is greater than the sum of its isolated components.
The notion of an entourage effect implicitly called into question the primacy of monomolecular medicine favored by pharmaceutical firms and government regulators. It also pointed beyond the canonical endocannabinoid system to a wider schema that encompassed more than a pair of receptors, their ligands, and related enzymes. By highlighting the interplay between endocannabinoids and other lipid signaling molecules, pioneers in the burgeoning field of cannabinoid science pushed the conceptual envelope and opened the door to new vistas of understanding human biology and physiology.
1999: TRP (“TRIP”) ION CHANNELS
Scientists developed research tools to probe and modulate various aspects of the endocannabinoid system. By administering synthetic “antagonist” compounds to block the CB1 receptor, scientists discerned that some of anandamide’s effects did not involve this receptor. In 1999, a team of European researchers reported in Nature that the ability of anandamide, a vasodilator, to relax blood vessels was mediated by its interaction with the “TRPV1” vanilloid receptor. Subsequent studies determined that 2-AG is also active at the TRPV1 receptor, which is instrumental in regulating core body temperature and inflammatory pain.
Read More below from projectcbd.org
About Wellness BioSciences Rx (WBRx)
Wellness BioSciences Rx (WBRx) is a global innovator in the delivery of science-based wellness consumables in partnership with health care providers directly to their respective patient base. Our products specialize in the development of the highest grade 100% USA-grown hemp oil rich in CBD, CBDA, CBG, CBN, CBC, and terpenes. WBRx was founded by Barry Cocheu and Sean Baker.